Introduction to Uranium Enrichment
Natural uranium contains only about 0.7% of the fissile isotope uranium-235 (U-235), which is required for nuclear power generation and weapons development 12. The remaining 99.3% consists primarily of uranium-238 (U-238), which does not contribute directly to the fission process 1.
To be useful in most commercial nuclear reactors, uranium must be “enriched” to contain 3-5% U-235, a process that separates isotopes based on their slight mass differences 34. For weapons-grade uranium, enrichment levels above 90% are typically required 5.
While gas centrifuge technology dominates today’s commercial uranium enrichment industry, several alternative methods have been developed throughout history 26. These alternatives operate on different physical principles but share the common goal of increasing the concentration of U-235 3.
Understanding these alternative enrichment technologies is crucial for nuclear policy, non-proliferation efforts, and the development of more efficient nuclear fuel cycles 78. Each method offers unique advantages and challenges in terms of efficiency, energy consumption, and proliferation risks 5.
Gaseous Diffusion: The First Commercial Method
Gaseous diffusion was the first economically viable uranium enrichment process and played a crucial role during the Manhattan Project and subsequent nuclear development 69. This technology dominated the commercial enrichment landscape for decades before being largely replaced by centrifuge technology 10.
The basic principle behind gaseous diffusion is remarkably simple: molecules containing the lighter U-235 isotope diffuse through barriers slightly faster than those containing the heavier U-238 96. This process relies on Graham’s Law of Diffusion, which states that the rate of diffusion is inversely proportional to the square root of molecular mass 10.
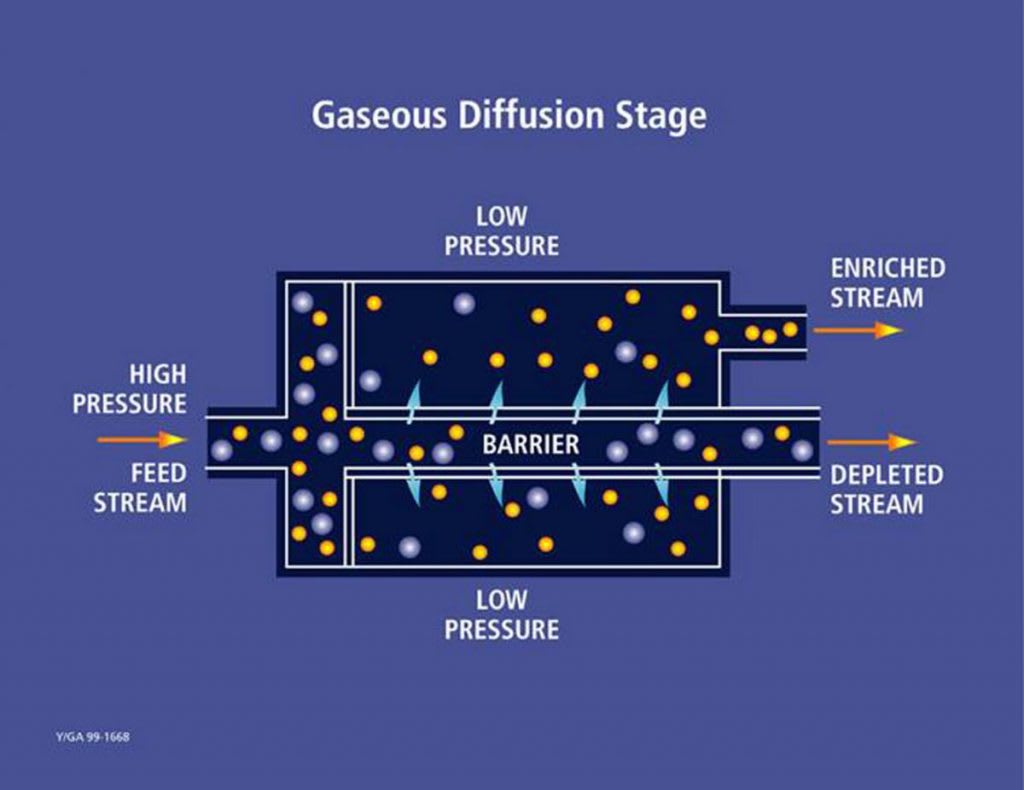
Diagram illustrating a single stage of the gaseous diffusion process for isotope separation.
In practice, uranium hexafluoride (UF₆) gas is pumped through semi-permeable membranes with microscopic pores 26. The lighter UF₆ molecules containing U-235 pass through these barriers slightly more readily than their heavier U-238 counterparts 610.
Each pass through a barrier produces only a very slight enrichment, with the separation factor typically around 1.004 23. This means that hundreds of stages are required in sequence to achieve commercially useful enrichment levels 10.
A single diffusion stage consists of a compressor, a cooler to remove the heat of compression, and a diffuser containing the barrier 64. The enriched stream from each stage becomes the feed for the next higher stage, while the depleted stream is recycled back to the next lower stage, creating a cascade 3.
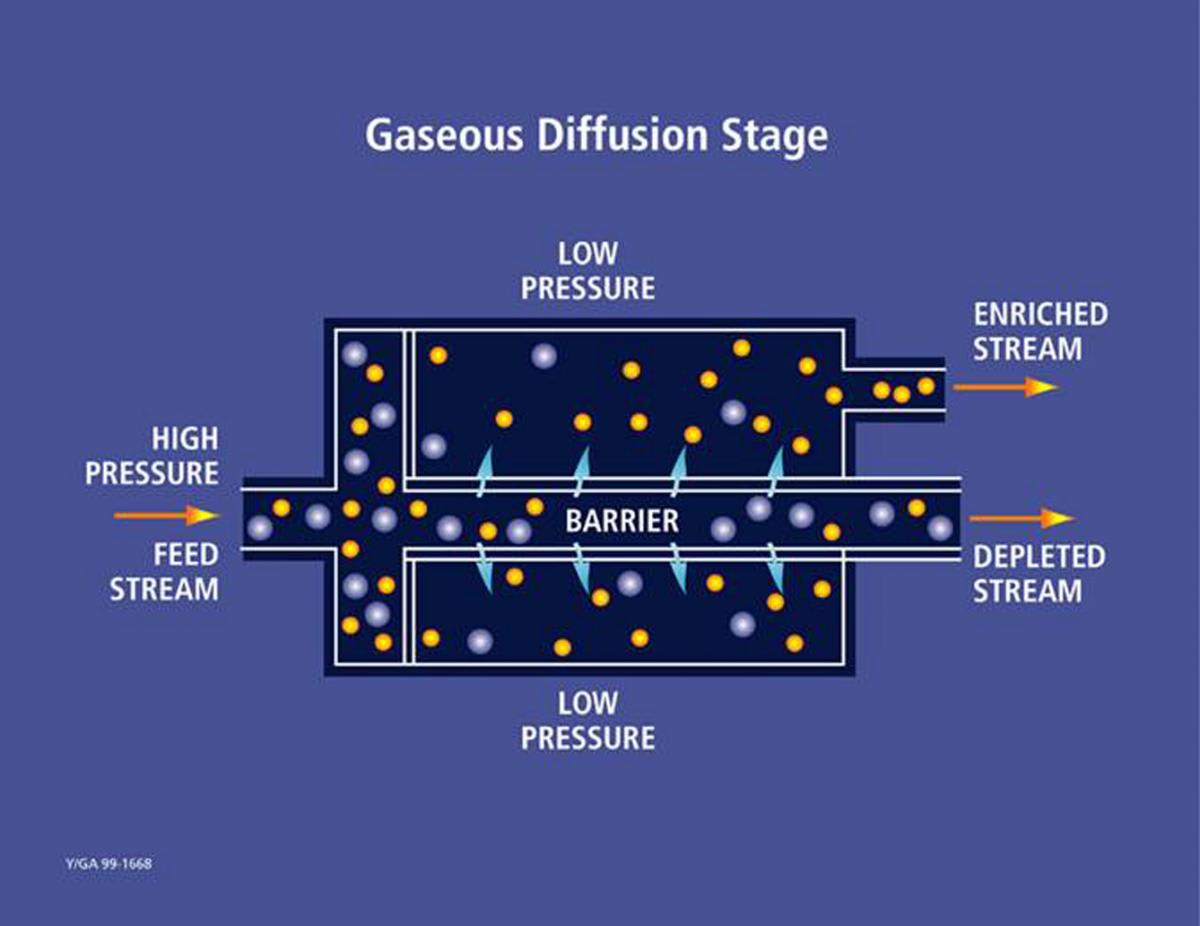
Diagram illustrating a single stage of the gaseous diffusion process for isotope separation.
Gaseous diffusion plants were massive industrial facilities requiring enormous energy inputs 310. The Oak Ridge K-25 plant in Tennessee, completed in 1945, was once the world’s largest building under one roof and consumed huge amounts of electricity 10.
The energy intensity of gaseous diffusion became its major drawback - requiring approximately 50 times more electricity than centrifuge technology for the same separation work 83. This led to the gradual phase-out of diffusion plants worldwide 10.
The last major gaseous diffusion plant in the United States, located in Paducah, Kentucky, ceased operations in 2013 311. France’s Georges Besse I plant, the last major diffusion facility outside the US, shut down in 2012 11.
Laser Enrichment: Next-Generation Technology
Laser enrichment represents a revolutionary approach to uranium isotope separation that exploits the slight differences in the absorption spectra of different isotopes 37. This technology promises higher efficiency with significantly lower energy requirements compared to traditional methods 1213.
Unlike mechanical separation methods, laser enrichment uses precisely tuned lasers to selectively excite or ionize atoms or molecules containing U-235 but not U-238 314. These excited atoms can then be separated through various collection methods 13.
Several laser-based approaches have been developed, with the most prominent being Atomic Vapor Laser Isotope Separation (AVLIS), Molecular Laser Isotope Separation (MLIS), and Separation of Isotopes by Laser Excitation (SILEX) 1315.
AVLIS uses lasers tuned to specific frequencies that can ionize atomic uranium-235 vapor but not uranium-238 157. The ionized U-235 atoms can then be attracted to a charged collector plate and separated from the neutral U-238 atoms 16.
MLIS works with uranium in molecular form, typically as uranium hexafluoride gas 1714. The process uses infrared lasers to vibrationally excite UF₆ molecules containing U-235, followed by ultraviolet photons to cause photodissociation, producing UF₅ and a free fluorine atom 1718.
The SILEX process, developed in Australia, is considered a third-generation enrichment technology and uses laser light to excite UF₆ molecules containing U-235 1419. While the specific details remain classified, the technology reportedly uses carbon dioxide lasers converted to the appropriate wavelength through a Raman cell filled with hydrogen 20.
Global Laser Enrichment (GLE), a joint venture between Silex Systems and Cameco Corporation, is working to commercialize the SILEX technology in the United States 1421. Recent tests have demonstrated full-scale laser systems operating reliably under plant-like conditions 1922.
Laser enrichment offers several potential advantages over traditional methods, including higher separation factors, lower energy consumption, and smaller facility footprints 2312. However, these same advantages raise proliferation concerns, as the technology could potentially be more difficult to detect and monitor 12.
A key milestone was reached in September 2012 when the US Nuclear Regulatory Commission granted a license for the construction and operation of a commercial laser enrichment facility 321. The technology demonstration is on track for completion in the mid-2020s, with commercial operations potentially beginning around 2027-2028 2122.
Electromagnetic Separation: The Manhattan Project Method
Electromagnetic isotope separation was one of the earliest methods developed for uranium enrichment, playing a crucial role in the Manhattan Project during World War II 224. This technology utilized devices called calutrons, which were essentially large mass spectrometers designed specifically for uranium separation 2425.

An interior view of a Calutron facility, showcasing the circular arrangement of units used for electromagnetic uranium isotope separation.
Named after the California University Cyclotron (calutron), these devices were developed by Ernest Lawrence and his team at Berkeley 2426. The technology was deployed on an industrial scale at the Y-12 facility in Oak Ridge, Tennessee, producing the enriched uranium used in the “Little Boy” atomic bomb dropped on Hiroshima 24.
Calutrons work by vaporizing and ionizing uranium metal, then accelerating these ions through a magnetic field 2425. The magnetic field causes the ions to travel in a curved path, with the lighter U-235 ions bending more sharply than the heavier U-238 ions 25.
Collectors placed at different positions capture the separated streams of uranium isotopes 2527. While effective, the process was extremely energy-intensive, requiring enormous amounts of electricity and significant quantities of copper or silver wire for the electromagnetic coils 24.
In an interesting historical note, during World War II, silver from the U.S. Treasury was borrowed to make the electromagnetic coils when copper was in short supply 2728. After the war, the silver was recovered and returned 28.
The electromagnetic separation method was largely abandoned after the war in favor of more efficient techniques like gaseous diffusion 2427. Today, calutrons are primarily used for separating stable isotopes for scientific, medical, and industrial applications rather than for uranium enrichment 27.
Thermal Diffusion: Heat-Driven Separation
Thermal diffusion is another historical method for uranium enrichment that exploits how temperature gradients affect isotope distribution 2930. This process was used at the S-50 plant in Oak Ridge during the Manhattan Project as part of the uranium enrichment effort 31.

Exterior view of the S-50 thermal diffusion plant, a facility used for uranium enrichment during the Manhattan Project.
In liquid thermal diffusion, uranium hexafluoride is placed between two vertical concentric pipes 1032. The inner pipe is heated with steam while the outer pipe is cooled with water, creating a temperature gradient across the UF₆ 3233.
Through a phenomenon called the “Soret effect,” the lighter molecules containing U-235 tend to migrate toward the hotter surface, while the heavier U-238 molecules concentrate near the cooler surface 2933. Convection currents then carry the enriched U-235 to the top of the column where it can be collected 33.
The S-50 thermal diffusion plant was constructed in just 69 days in 1944 but was only ever used as a preliminary enrichment step before further processing at other facilities 3129. The plant contained 2,142 columns, each standing 48 feet tall 31.
Despite its initial usefulness, thermal diffusion proved less efficient than other methods and consumed too much power relative to its output 3130. The S-50 plant was deactivated in 1946 and demolished a few years later 31.
Interestingly, the thermal diffusion process was originally developed by Philip Abelson for the U.S. Navy, which was interested in nuclear propulsion for submarines 3233. The technology was later incorporated into the Manhattan Project when its potential for uranium enrichment was recognized 33.
Aerodynamic Processes: Motion-Based Separation
Aerodynamic enrichment processes represent another category of uranium separation techniques that rely on the principles of fluid dynamics and slight mass differences between isotopes 434. These methods include the separation nozzle process and the vortex tube separation process 34.
In these processes, a mixture of uranium hexafluoride gas and a lighter carrier gas (typically hydrogen or helium) is forced through specially designed nozzles or tubes at high speeds 3435. The centrifugal forces created by the curved paths separate the isotopes based on their different masses 34.
The separation nozzle process, developed by E.W. Becker in Germany, directs the gas mixture along a curved wall at high velocity 3435. The heavier U-238 molecules move preferentially toward the wall, allowing a knife edge to split the flow into enriched and depleted streams 34.
The vortex tube separation process, notably used in South Africa’s nuclear program, involves injecting the UF₆-hydrogen mixture tangentially into a tube that tapers to small exit apertures 342. The resulting vortex creates centrifugal forces that separate the isotopes 34.
Both processes can be considered as “non-rotating centrifuges” since they rely on pressure gradients to create separation effects similar to those in centrifuge technology 344. However, they typically require complex arrangements of cascading units to minimize energy consumption 2.
Research at Boston College demonstrated a variation called the “jet membrane” concept, which showed promise for uranium enrichment with potentially higher efficiency than conventional methods 35. Their experiments indicated separation factors significantly higher than those achievable with gaseous diffusion 35.
While aerodynamic processes have been implemented in countries like South Africa and Brazil, none are currently in commercial use for uranium enrichment 234. The high energy requirements and complexity of these systems have limited their industrial adoption 34.
Chemical Exchange: Solution-Based Separation
Chemical exchange enrichment represents a fundamentally different approach that exploits slight differences in chemical reactions between uranium isotopes 3637. Unlike most other methods, this process operates with uranium in liquid rather than gaseous form 37.
The Asahi Chemical Exchange Process (ACEP), developed in Japan, is one of the most notable examples of this technology 3638. This process utilizes the principle that uranium isotopes distribute differently between two chemical forms in a liquid-liquid exchange system 3837.
In ACEP, uranium moves between two immiscible liquid phases—one aqueous and one organic 3937. The slightly different chemical behavior of U-235 and U-238 causes one isotope to preferentially concentrate in one phase over the other 38.
The separation process typically consists of several tens to hundreds of separation towers arranged to create a multistage system 37. Unlike conventional cascade arrangements, ACEP is designed to produce both enriched and depleted uranium streams from a single module without requiring a complex cascade 37.
Chemical exchange methods were previously considered impractical for large-scale uranium enrichment 3836. However, improvements reported in both Japan and France have sparked renewed interest in these techniques 38.
Advocates highlight several advantages of chemical exchange, including resistance to nuclear proliferation concerns and effective utilization of uranium resources 3837. The technology is also noted for being relatively simple to maintain and operate compared to some alternatives 37.
Research continues to improve the efficiency and practicality of chemical exchange methods 3836. The approach remains a potential alternative enrichment pathway, particularly as the nuclear industry seeks more proliferation-resistant technologies 36.
Plasma Separation: Cutting-Edge Research
Plasma separation represents one of the more experimental approaches to uranium enrichment that leverages principles from plasma physics and electromagnetism 4041. This method uses the different behaviors of ionized uranium isotopes in strong electromagnetic fields 40.
In the plasma separation process (PSP), a uranium feed plate serves as the source of neutral uranium atoms, which are then vaporized through a process called sputtering 40. Electrons collide with these neutral atoms, creating a plasma containing U-235 and U-238 ions 40.
This plasma is subjected to a strong magnetic field generated by superconducting magnets surrounding the vacuum chamber 4041. The magnetic field causes the ions to move in helical paths, with the lighter U-235 ions spiraling faster and having a higher cyclotron frequency 40.
As the plasma flows through the chamber, it passes through an electric field oscillating at the same frequency as the cyclotron frequency of U-235 ions 40. This selective resonance causes the U-235 ions to increase their orbital radius while having minimal effect on U-238 ions 40.
The process culminates with the plasma flowing through a collector system resembling a venetian blind 40. The large-orbit U-235 ions are more likely to deposit on the slats, while the depleted plasma accumulates on an end plate 40.
A variation called the plasma centrifuge, studied by researchers at Hitachi Ltd., uses electromagnetic acceleration to create extremely high rotational velocities in the plasma 41. Their analysis suggested potential rotational speeds of 2.6 km/sec, creating centrifugal forces strong enough to enable a more compact separation system than conventional mechanical centrifuges 41.
Plasma separation techniques remain largely experimental and have not been deployed commercially for uranium enrichment 40. However, advances in superconducting magnets and plasma physics continue to improve the theoretical potential of these methods 4041.
Recent Developments and Future Outlook
The uranium enrichment landscape continues to evolve with significant recent developments across multiple technologies 4221. The past decade has seen particular advancement in laser enrichment, with Silex Systems achieving important milestones in scaling up their technology 2119.
In 2022, Silex successfully manufactured and tested the first full-scale laser system module for uranium enrichment, demonstrating reliable operation at commercial scale for extended periods 1922. This equipment is being installed at Global Laser Enrichment’s facility in Wilmington, North Carolina 22.
The U.S. Department of Energy has shown strong support for next-generation enrichment technologies, selecting Global Laser Enrichment as one of six companies to receive funding under its Low Enriched Uranium Program 4321. This initiative aims to reduce American dependence on Russian nuclear fuels 43.
Researchers at the Chinese Academy of Sciences have developed an innovative method for pre-enriching uranium from seawater using membrane filtration 4445. This approach could potentially unlock access to the estimated 4.5 billion tons of uranium dissolved in the world’s oceans 44.
In 2024, an Australian company called Ubaryon reported achieving a separation factor approximately three times higher than the enrichment factor in their proprietary chemical separation process for uranium isotopes 46. This advancement marks a key milestone in developing an alternative commercial enrichment technology 46.
BWX Technologies announced in April 2025 the acquisition of land in Oak Ridge, Tennessee, to support the development of domestic uranium enrichment capabilities using advanced centrifuge technology 42. This initiative aims to create hundreds of new jobs and establish a reliable domestic supply of enriched uranium for defense purposes 42.
The latest “Red Book” report from the OECD Nuclear Energy Agency and the International Atomic Energy Agency indicates that current uranium resources are sufficient to meet nuclear capacity needs through 2050 and beyond 47. However, the report stresses that further development of resources will still be required to meet long-term demand 47.
Industry analysts predict that the global shift toward clean energy solutions may drive increased demand for enrichment services in coming decades 4743. This trend, coupled with concerns about supply chain resilience, is likely to encourage continued investment in diverse enrichment technologies 4342.
Comparing Enrichment Technologies
Each uranium enrichment method offers distinct advantages and faces unique challenges in terms of efficiency, energy consumption, and technical complexity 35. Understanding these differences is crucial for evaluating their commercial viability and proliferation implications 512.
Gaseous diffusion, while historically important, requires enormous amounts of electricity—approximately 50 times more than centrifuge technology for equivalent output 83. Its large facility footprint and high energy costs ultimately led to its commercial obsolescence 3.
Gas centrifuge technology, currently dominant in the market, offers significantly better energy efficiency with a separation factor of 1.3-1.6 per stage compared to gaseous diffusion’s 1.005 4849. This translates to about 98% less energy consumption for the same separative work 11.
Laser enrichment methods potentially offer even greater efficiency than centrifuge technology, with higher separation factors and lower energy requirements 1323. However, these same advantages raise proliferation concerns since the technology could enable smaller, harder-to-detect facilities 12.
Electromagnetic separation, while effective, proved extremely energy-intensive, requiring up to a terajoule of energy to produce just one gram of highly enriched isotope 27. This energy penalty makes it economically nonviable for large-scale uranium enrichment 24.
Thermal diffusion and aerodynamic processes both suffer from high energy consumption relative to their output 3134. Neither has proven commercially competitive with centrifuge technology for uranium enrichment 3134.
Chemical exchange methods like ACEP offer potential advantages in proliferation resistance but historically have faced challenges in separation efficiency 3837. Recent improvements have sparked renewed interest in these techniques 38.
From a technical perspective, gaseous diffusion requires approximately 1,400 stages to produce reactor-grade fuel, while centrifuges need only 10-20 stages 49. Laser enrichment could potentially achieve similar results with even fewer separation stages 13.
Energy requirements vary dramatically between technologies, with gaseous diffusion consuming about 2,400 kWh per SWU (separative work unit), gas centrifuges using 50-60 kWh per SWU, and laser enrichment potentially requiring as little as 10-20 kWh per SWU 4812.
Conclusion
The evolution of uranium enrichment technology represents a fascinating intersection of physics, chemistry, and engineering ingenuity 12. While gas centrifuge technology currently dominates the commercial landscape, alternative methods have played crucial historical roles and may yet shape the future of nuclear fuel production 23.
Gaseous diffusion, the workhorse of the early nuclear era, has now been retired from commercial service after decades of operation 311. Its replacement by more efficient centrifuge technology demonstrates how economic factors ultimately drive industrial adoption 11.
Laser enrichment stands at the threshold of potential commercial deployment, promising higher efficiency and lower energy costs 2221. The ongoing development of the SILEX technology could represent the next major shift in enrichment technology if successfully scaled up 1921.
Other methods like electromagnetic separation, thermal diffusion, and aerodynamic processes remain primarily of historical interest, though they continue to inform scientific understanding of isotope separation 243134. Meanwhile, more experimental approaches like plasma separation and chemical exchange offer alternative pathways that may yet find specialized applications 4038.
The diversification of enrichment technologies carries important implications for nuclear non-proliferation efforts, energy security, and the economics of nuclear power 512. Each advancement must be evaluated not only for its technical merits but also for its broader impact on international security 12.
As global energy demands grow and nations seek low-carbon electricity sources, uranium enrichment will remain a critical component of the nuclear fuel cycle 4742. The technologies that efficiently and securely provide this service will play an essential role in shaping our energy future 4243.
Footnotes
-
https://world-nuclear.org/information-library/nuclear-fuel-cycle/conversion-enrichment-and-fabrication/uranium-enrichment ↩ ↩2 ↩3
-
https://en.wikipedia.org/wiki/Enriched_uranium ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8 ↩9 ↩10
-
https://www.nrc.gov/materials/fuel-cycle-fac/ur-enrichment.html ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8 ↩9 ↩10 ↩11 ↩12 ↩13 ↩14 ↩15
-
https://geoinfo.nmt.edu/resources/uranium/enrichment.html ↩ ↩2 ↩3 ↩4
-
https://www.numberanalytics.com/blog/ultimate-uranium-enrichment-guide ↩ ↩2 ↩3 ↩4 ↩5
-
https://energyeducation.ca/encyclopedia/Gaseous_diffusion_uranium_enrichment ↩ ↩2 ↩3 ↩4 ↩5 ↩6
-
https://www.scitechnol.com/download.php?download=peer-review-pdfs%2Flaser-enrichment-advancements-and-applications-in-uranium-processing-rFYg.pdf ↩ ↩2 ↩3
-
https://davidson.weizmann.ac.il/en/online/sciencepanorama/understanding-uranium-enrichment ↩ ↩2
-
https://fluoridealert.org/news/uranium-enrichment-gaseous-diffusion-process/ ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8
-
https://www.cameco.com/uranium_101/fuel-processing/enrichment/ ↩ ↩2 ↩3 ↩4 ↩5
-
https://www.newscientist.com/article/dn20819-briefing-security-fears-over-laser-enriched-uranium/ ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8
-
https://en.wikipedia.org/wiki/Separation_of_isotopes_by_laser_excitation ↩ ↩2 ↩3 ↩4 ↩5
-
https://www.silex.com.au/silex-technology/silex-uranium-enrichment-technology/ ↩ ↩2 ↩3 ↩4
-
https://en.wikipedia.org/wiki/Atomic_vapor_laser_isotope_separation ↩ ↩2
-
https://en.wikipedia.org/wiki/Molecular_laser_isotope_separation ↩ ↩2
-
http://library.sciencemadness.org/lanl1_a/lib-www/pubs/00416662.pdf ↩
-
https://www.aumanufacturing.com.au/silex-tests-full-scale-laser-destined-for-uranium-enrichment ↩ ↩2 ↩3 ↩4 ↩5
-
https://everything.membrane.com/nuclear-energy-laser-uranium-enrichment/ ↩
-
https://www.world-nuclear-news.org/Articles/GLE-laser-enrichment-on-track-for-2024-demonstrati ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8
-
https://enertherm-engineering.com/laser-beams-nuclear-dreams-can-uranium-tests-unlock-unlimited-power/ ↩ ↩2
-
https://en.wikipedia.org/wiki/Calutron ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8 ↩9
-
https://www.osti.gov/opennet/manhattan-project-history/Processes/UraniumSeparation/electromagnetic.html ↩
-
https://physicsworld.com/a/laser-shines-a-new-light-on-isotope-separation/ ↩ ↩2 ↩3 ↩4 ↩5
-
https://ahf.nuclearmuseum.org/ahf/history/breakthroughs-1942/ ↩ ↩2
-
https://en.wikipedia.org/wiki/S-50_(Manhattan_Project) ↩ ↩2 ↩3
-
https://chem.libretexts.org/Ancillary_Materials/Exemplars_and_Case_Studies/Case_Studies/Nuclear_Energy_for_Today’s_World/09._Uranium_Enrichment ↩ ↩2
-
https://www.nps.gov/places/s-50-liquid-thermal-diffusion-plant.htm ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8
-
https://ahf.nuclearmuseum.org/ranger/tour-stop/s-50-plant/ ↩ ↩2 ↩3
-
https://ahf.nuclearmuseum.org/ahf/history/isotope-separation-methods/ ↩ ↩2 ↩3 ↩4 ↩5
-
https://www.globalsecurity.org/wmd/intro/u-aerodynamic.htm ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8 ↩9 ↩10 ↩11 ↩12 ↩13 ↩14
-
https://www.osti.gov/biblio/6873652-uranium-enrichment-chemical-exchange-process-acep-process ↩ ↩2 ↩3 ↩4 ↩5
-
https://www.osti.gov/etdeweb/biblio/6026542 ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8 ↩9
-
https://www.osti.gov/biblio/6873652 ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8 ↩9 ↩10
-
https://www.sipri.org/sites/default/files/files/books/SIPRI83Krass/SIPRI83Krass06.pdf ↩
-
https://www.globalsecurity.org/wmd/intro/u-plasma.htm ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8 ↩9 ↩10 ↩11 ↩12 ↩13
-
https://www.jstage.jst.go.jp/article/jnst1964/10/10/10_10_626/_pdf ↩ ↩2 ↩3 ↩4 ↩5
-
https://www.bwxt.com/bwxt-moving-forward-to-support-national-security-domestic-uranium-enrichment-mission/ ↩ ↩2 ↩3 ↩4 ↩5 ↩6
-
https://www.aumanufacturing.com.au/silex-systems-to-become-significant-uranium-enricher ↩ ↩2 ↩3 ↩4 ↩5
-
https://phys.org/news/2022-06-method-enrich-uranium-seawater.html ↩ ↩2
-
https://www.greencarcongress.com/2022/05/20220529-uranium.html ↩
-
https://www.ans.org/news/2025-04-10/article-6925/latest-red-book-stresses-need-for-boosts-in-uranium-development/ ↩ ↩2 ↩3 ↩4
-
https://www.belfercenter.org/sites/default/files/legacy/files/uploads/Enrichment-of-Uranium-and-Production-of-Plutonium.pdf ↩ ↩2