The transformation of uranium from its concentrated powder form into a gaseous compound represents one of the most critical steps in nuclear fuel preparation. This conversion process, which transforms yellowcake uranium into uranium hexafluoride (UF6) gas, serves as the essential bridge between uranium mining operations and the enrichment facilities that prepare reactor fuel. The process involves sophisticated chemical engineering, precise temperature control, and stringent safety protocols to convert thousands of tons of uranium concentrate annually into the specialized gaseous form required for isotopic separation. Understanding this conversion process reveals the complex industrial infrastructure underlying modern nuclear energy production and highlights the technical challenges involved in preparing uranium for its eventual use in power generation.

Metal drums containing yellowcake uranium powder, marked with radioactive hazard symbols, are stored on wooden pallets.
Understanding Yellowcake: The Starting Material
Composition and Characteristics
Yellowcake uranium represents the concentrated uranium oxide powder that emerges from uranium milling operations. This material, chemically designated as U3O8, contains approximately 75% uranium by weight, making it a highly concentrated uranium powder with around 750 kg of uranium oxide per tonne1. Despite its name suggesting a yellow appearance, yellowcake can vary significantly in color from yellow to orange to dark green or blackish, depending on the temperature at which the material is dried and the level of impurities present2.
The variation in color reflects different levels of hydration and impurities that result from various drying temperatures, with higher temperatures producing darker and less soluble material2. This uranium concentrate represents the endpoint of the milling process, where uranium ore has been processed to separate the uranium from waste rock and other minerals through chemical leaching operations.
Production Methods and Sources
Modern yellowcake production relies heavily on in-situ leaching methods rather than conventional mining and milling techniques. In-situ leaching involves circulating groundwater with high oxygen content through uranium ore deposits, extracting uranium without major ground disturbance2. The uranium-laden solution is then pumped to the surface where it undergoes ion exchange processes using resin beads that attract uranium from the solution.
Conventional milling methods, while less commonly used today, involved crushing ore and creating a slurry of fine particles suspended in water, followed by leaching with sulfuric acid to dissolve uranium oxides2. The remaining rock and minerals that do not dissolve in the acid become tailings, while the uranium-rich solution undergoes further processing to precipitate the yellowcake concentrate.

Metal drums labeled with radioactive symbols, containing yellowcake uranium concentrate, stored on wooden pallets.
Radioactivity and Safety Considerations
Yellowcake uranium exhibits only mild radioactivity due to its composition of primarily natural uranium. The radiation level one meter from a drum of freshly-processed uranium oxide measures approximately half the radiation experienced from cosmic rays during a commercial jet flight2. This relatively low radioactivity stems from uranium’s composition of approximately 99.289% U-238, which has a half-life of roughly 4 billion years.
The long half-life of U-238 results in very low radioactivity, as substances with shorter half-lives are more radioactive than those with longer half-lives2. Despite this relatively low radioactivity, yellowcake still requires proper handling procedures and storage in appropriate containers marked with radioactive material warnings to ensure worker safety and regulatory compliance.
The Chemical Conversion Process
Overview of Conversion Requirements
The conversion of yellowcake into uranium hexafluoride serves as an essential prerequisite for uranium enrichment operations. Since all enrichment technologies used on an industrial scale require a gaseous medium, uranium hexafluoride is utilized widely because of its unique chemical properties, particularly its ability to change state over a relatively small range of temperatures3. This conversion process transforms the solid uranium oxide concentrate into a compound suitable for gaseous diffusion or gas centrifuge enrichment processes.
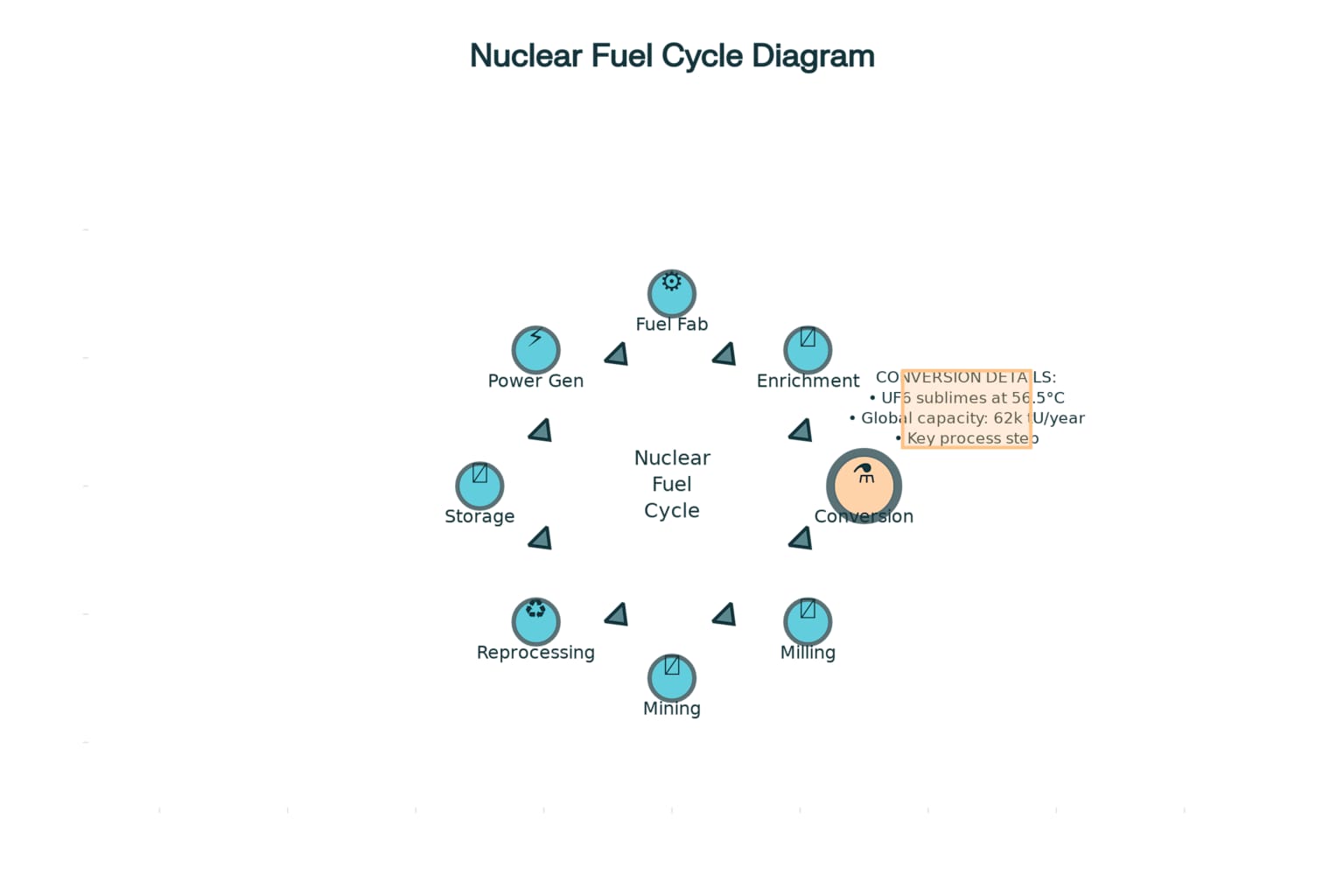
Nuclear Fuel Cycle with Emphasis on Uranium Conversion
Uranium hexafluoride, commonly referred to as “hex,” provides the ideal medium for enrichment because it can conveniently be used as a gas for processing while also being transportable and storable as a solid or liquid without requiring extreme pressures or temperatures3. The conversion process must achieve high purity levels to ensure that subsequent enrichment operations proceed efficiently and safely.
Step-by-Step Chemical Processes
The conversion of yellowcake to uranium hexafluoride follows a carefully controlled multi-step chemical process. Initially, yellowcake powder undergoes dissolution in nitric acid to form an aqueous solution containing uranyl nitrate4. This dissolution step prepares the uranium for subsequent chemical transformations by converting the oxide form into a more reactive chemical state.
The second step involves reduction using hydrogen gas to convert the aqueous solution of U3O8 and UO3 to uranium dioxide according to the reaction: U3O8 + 2 H2 → 3 UO2 + 2 H2O4. This reduction step standardizes the uranium to the dioxide form, providing a consistent starting material for the fluorination reactions that follow.
The third step consists of a kiln reaction where uranium dioxide reacts with hydrofluoric acid to form uranium tetrafluoride: UO2 + 4 HF → UF4 + 2 H2O4. This hydrofluorination process introduces fluorine atoms into the uranium compound, creating an intermediate product known as “green salt” due to its characteristic color5.
The final step involves feeding uranium tetrafluoride into a fluidized bed reactor with fluorine gas to produce uranium hexafluoride: UF4 + F2 → UF64. This fluorination reaction represents the culmination of the conversion process, creating the final gaseous product ready for enrichment operations.
Alternative Process Routes
Some conversion facilities employ alternative process routes depending on their equipment configuration and operational requirements. The main wet process, used by facilities in Canada, France, China, and Russia, involves dissolving the uranium concentrate in nitric acid and feeding the resulting uranyl nitrate solution into a countercurrent solvent extraction process using tributyl phosphate dissolved in kerosene or dodecane6.

Large blue industrial processing unit, likely a desublimer or regasification vessel, used in uranium conversion.
The uranium gets collected by the organic extractant, from which it can be washed out by dilute nitric acid solution and then concentrated by evaporation6. The solution then undergoes calcination in a fluidized bed reactor to complete the conversion to uranium hexafluoride. This wet process offers advantages in terms of purification capabilities and contamination control.
Properties and Characteristics of Uranium Hexafluoride
Physical Properties
Uranium hexafluoride exhibits unique physical properties that make it ideally suited for uranium enrichment processes. At room temperature, UF6 exists as a colorless, crystalline solid with significant vapor pressure7. The compound sublimes directly from solid to gas at 56.5°C at atmospheric pressure, eliminating the need for intermediate liquid phases during typical processing operations78.
The triple point of uranium hexafluoride occurs at 64.02°C and 1.497 atm, representing the unique temperature and pressure conditions where solid, liquid, and gas phases can coexist in equilibrium9. Above the triple point pressure, UF6 can exist as a liquid, which proves useful for certain processing applications where pumping liquid material provides operational advantages.
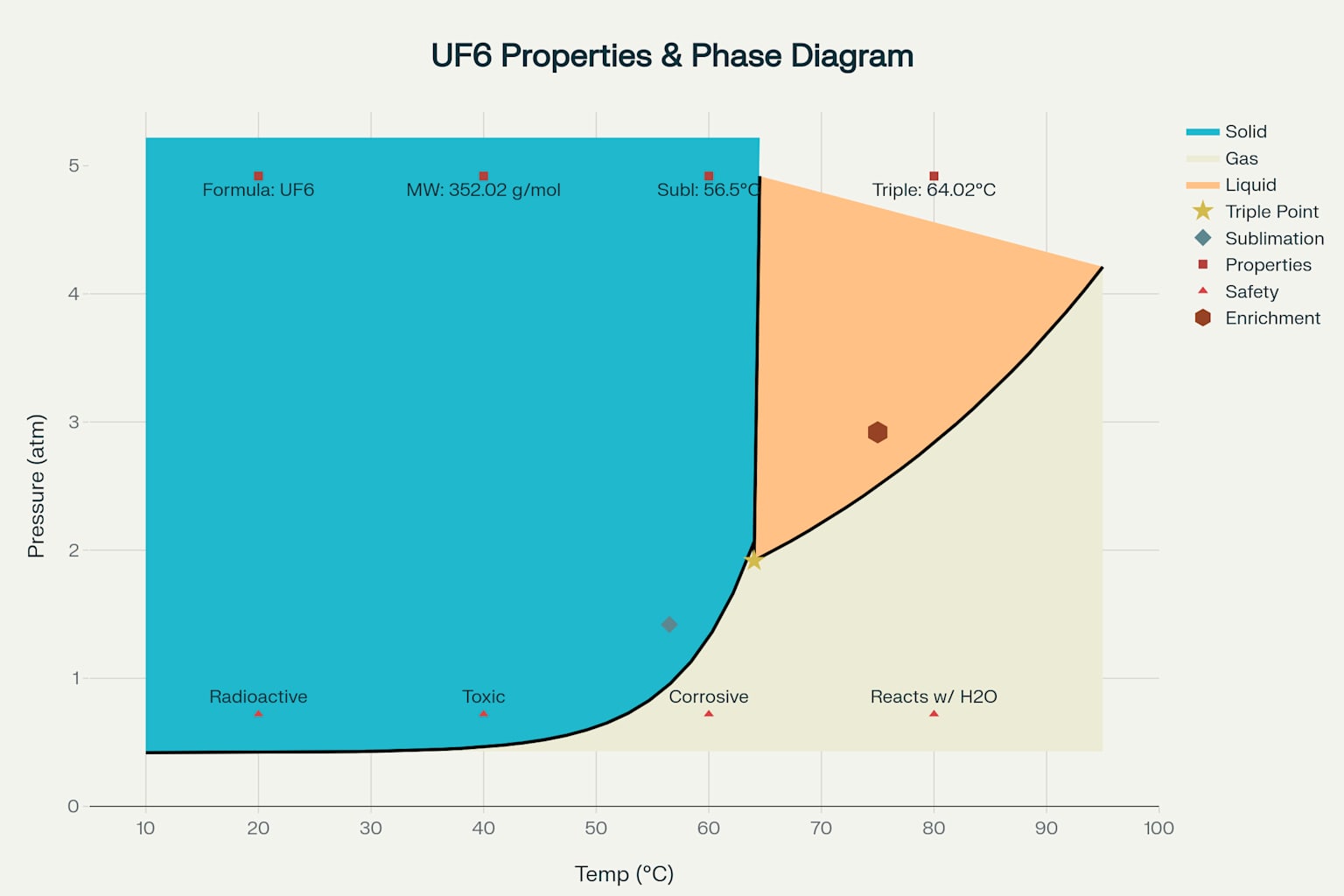
Uranium Hexafluoride (UF6) Properties and Characteristics
Chemical Behavior and Reactivity
Uranium hexafluoride demonstrates mild oxidizing properties and functions as a Lewis acid, as evidenced by its ability to bind and form heptafluorouranate(VI) complexes7. The compound reacts vigorously with water, releasing hydrofluoric acid according to the reaction that produces mainly hydrogen fluoride and water-soluble uranyl fluoride10. This water reactivity necessitates careful moisture control in all handling and processing operations.
The compound remains stable in dry air conditions but shows reactivity with various materials including aluminum, where it forms a protective surface layer of AlF3 that resists further reaction7. This selective reactivity influences the choice of materials used in processing equipment and storage containers throughout the conversion and enrichment facilities.
Storage and Transport Considerations
Uranium hexafluoride requires specialized storage and transport containers designed to accommodate its unique properties and safety requirements. The material is typically stored in cylindrical steel containers ranging from small 2.5-ton product cylinders to large 14-ton storage and transport cylinders1112. These containers must withstand the chemical corrosivity of UF6 while providing secure containment during storage and transportation.

Aerial view of a train derailment in Rockingham, North Carolina, in 1977, involving scattered freight and four uranium hexafluoride (UF6) cylinders.
The containers undergo rigorous testing to ensure structural integrity under various temperature and pressure conditions. Large-diameter cylinders used for depleted uranium hexafluoride storage have been constructed with both thick-wall and thin-wall configurations, with wall thicknesses of 5/8-inch and 5/16-inch respectively13. These containers require careful handling to prevent damage from corrosion, stacking operations, and other mechanical stresses that could compromise their integrity.
Global Conversion Facilities and Infrastructure
Major Conversion Centers
The global uranium conversion industry operates through a limited number of specialized facilities concentrated in countries with significant nuclear sectors. According to current capacity estimates, the world’s primary conversion facilities include operations in Canada, China, France, Russia, and the United States, with a combined licensed capacity of approximately 62,000 metric tons of uranium annually6.
Cameco operates Canada’s conversion facility in Port Hope, Ontario, with a licensed capacity of 12,500 metric tons of uranium per year and production of approximately 10,600 metric tons in 20226. This facility represents one of the largest conversion operations globally and serves customers throughout the international nuclear fuel market.
China’s conversion capacity, estimated at around 15,000 metric tons annually, operates through facilities in Lanzhou and Hengyang under China National Nuclear Corporation6. These facilities support China’s expanding domestic nuclear reactor fleet while also participating in international uranium conversion markets.
United States Conversion Infrastructure
The United States maintains one commercial uranium conversion facility, operated by Honeywell International Inc. in Metropolis, Illinois. This facility, built in 1958, represents the only uranium hexafluoride conversion facility in the United States and has an annual conversion capacity of approximately 15,000 metric tons of uranium14. The plant accounts for approximately 20% of worldwide conversion capacity and serves as a critical component of the global nuclear fuel supply chain.
ConverDyn, a general partnership between affiliates of Honeywell and General Atomics, serves as the exclusive agent for conversion sales from the Metropolis facility14. The plant processes U3O8 yellowcake received from uranium mines worldwide and produces uranium hexafluoride gas that is shipped to enrichment facilities for further processing into nuclear reactor fuel.
The facility currently operates in “idle-ready” status according to Nuclear Regulatory Commission information, reflecting market conditions and demand fluctuations in the global uranium conversion market11. This operational flexibility allows the facility to respond to changes in nuclear fuel demand while maintaining the capability to resume full production when market conditions warrant.
International Capacity and Distribution
France operates significant conversion capacity through Orano’s facilities at Pierrelatte and Malvesi, with a combined licensed capacity of 15,000 metric tons annually and production of approximately 8,900 metric tons in 20226. Orano has recently commissioned new conversion facilities, including the Philippe Coste plant at Tricastin, which began operations in 2018 and is expected to reach its rated output of 15,000 tons by 202115.
Russia maintains substantial conversion capacity through Rosatom facilities at Seversk, with an estimated licensed capacity of 12,500 metric tons annually and production of approximately 12,000 metric tons in 20226. These facilities support both domestic reactor requirements and international export markets, making Russia a significant player in the global conversion industry.
The geographic concentration of conversion facilities reflects the specialized nature of the technology and the significant capital investments required to establish and maintain these operations. The limited number of conversion facilities globally creates interdependencies in the nuclear fuel supply chain and emphasizes the importance of maintaining reliable international cooperation in nuclear fuel markets.
Safety and Environmental Considerations
Radiological and Chemical Hazards
Uranium conversion facilities face unique safety challenges due to the presence of both radiological and chemical hazards. The primary risks associated with conversion operations are more chemical than radiological, involving volatile and soluble chemicals including fluorine, hydrofluoric acid, and uranyl fluoride11. These chemical forms contribute to inhalation risks if releases occur during processing operations.
The conversion process utilizes hydrogen gas, which presents flammable and explosion hazards that require careful monitoring and control systems11. Nuclear criticality is not a significant hazard at conversion facilities because the nuclear material consists of natural uranium throughout the process, making criticality events impossible under normal operating conditions.
When uranium hexafluoride is released into the environment, it reacts with moisture in the air to produce hydrogen fluoride and uranyl fluoride compounds10. These reaction products present additional safety hazards beyond the radiological concerns, requiring comprehensive safety analyses that address both radiological and non-radiological risks.
Emergency Response and Containment
Conversion facilities must maintain comprehensive emergency response capabilities to address potential releases of uranium hexafluoride and associated hazardous chemicals. Large releases of hydrogen fluoride can potentially result in adverse off-site consequences, including serious injuries or fatalities, making emergency preparedness a critical safety consideration10.
The facilities implement defense-in-depth safety principles, with the first two levels of defense focusing on design features and operational procedures to reduce risks to appropriately low levels10. These safety measures include engineered containment systems, atmospheric monitoring, and trained emergency response teams capable of addressing various accident scenarios.
Conversion facilities operate under strict international regulations and oversight from national nuclear regulatory authorities. The regulatory framework addresses facility design, construction, operation, and decommissioning phases to ensure comprehensive safety throughout the facility lifecycle10.
Long-term Environmental Impacts
The environmental impacts of uranium conversion operations extend beyond immediate operational concerns to include long-term waste management and facility decommissioning challenges. Conversion facilities generate various waste streams containing uranium and other radioactive materials that require careful management and disposal in accordance with regulatory requirements.
Tailings disposal sites associated with uranium processing represent significant potential sources of contamination for thousands of years, with long-term risks that remain poorly defined despite improvements in waste management practices16. The effectiveness of modern uranium waste management facilities designed according to current best practices requires continued monitoring and evaluation over extended time periods.
Environmental monitoring programs at conversion facilities track air quality, water quality, soil contamination, and biological impacts to ensure that operations remain within acceptable environmental limits. These monitoring programs provide essential data for assessing the effectiveness of environmental protection measures and identifying potential areas for improvement in facility operations and waste management practices.
Conclusion
The conversion of yellowcake uranium to uranium hexafluoride gas represents a critical technological bridge in the nuclear fuel cycle, enabling the transformation of mined uranium into a form suitable for isotopic enrichment. This complex chemical process, involving multiple reaction steps and sophisticated industrial equipment, demonstrates the advanced engineering capabilities required to support modern nuclear energy production. The limited number of conversion facilities worldwide, concentrated in just a few countries, highlights both the specialized nature of this technology and the importance of international cooperation in maintaining reliable nuclear fuel supplies.
The safety and environmental considerations surrounding uranium conversion operations emphasize the need for continued vigilance in facility design, operation, and regulatory oversight. As nuclear energy continues to play an important role in global electricity generation and climate change mitigation efforts, the uranium conversion industry must maintain the highest standards of safety and environmental protection while meeting the growing demand for nuclear fuel. The technical sophistication and safety protocols developed for uranium conversion operations provide a foundation for continued advancement in nuclear fuel cycle technologies and contribute to the safe and reliable operation of nuclear power systems worldwide.
Footnotes
-
https://www.nrc.gov/materials/fuel-cycle-fac/ur-conversion.html ↩
-
https://www.enec.gov.ae/discover/fueling-the-barakah-plant/the-nuclear-fuel-cycle/ ↩ ↩2 ↩3 ↩4 ↩5 ↩6
-
https://www.nuclear-power.com/nuclear-power-plant/nuclear-fuel/nuclear-fuel-cycle/uranium-fuel-cycle/ ↩ ↩2
-
https://energyeducation.ca/encyclopedia/Yellowcake ↩ ↩2 ↩3 ↩4
-
https://www.centrusenergy.com/learn-more/nuclear-fuel-cycle/enrichment/ ↩
-
https://www.nuclear-power.com/nuclear-power-plant/nuclear-fuel/nuclear-fuel-cycle/yellowcake-u3o8/ ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7
-
https://en.wikipedia.org/wiki/Uranium_hexafluoride ↩ ↩2 ↩3 ↩4
-
https://resources.inmm.org/system/files/patram_proceedings/2001/33016.PDF ↩
-
https://pubchem.ncbi.nlm.nih.gov/compound/Uranium-hexafluoride ↩ ↩2 ↩3 ↩4 ↩5
-
https://world-nuclear.org/information-library/nuclear-fuel-cycle/conversion-enrichment-and-fabrication/conversion-and-deconversion ↩ ↩2 ↩3 ↩4
-
https://en.wikipedia.org/wiki/Honeywell_Uranium_Hexafluoride_Processing_Facility ↩
-
https://www.acs.org/molecule-of-the-week/archive/u/uranium-hexafluoride.html ↩ ↩2
-
https://energyeducation.ca/encyclopedia/Uranium_hexafluoride ↩
-
https://www-pub.iaea.org/MTCD/Publications/PDF/PUB2038_web.pdf ↩